Adeno-Associated Virus (AAV), also known as Adeno-Associated viral vector, is a widely used and versatile technology in the field of gene therapy. AAV is a small, non-pathogenic virus that belongs to the family of parvoviruses. It has gained significant attention as viral vectors for delivering therapeutic genes into target cells, making it an essential component of many gene therapy treatments. AAV is also considered the safest and most effective gene delivery vehicles for gene delivery for the treatment of a variety of human diseases.
At OranssiBio, we provide AAV with the following features:
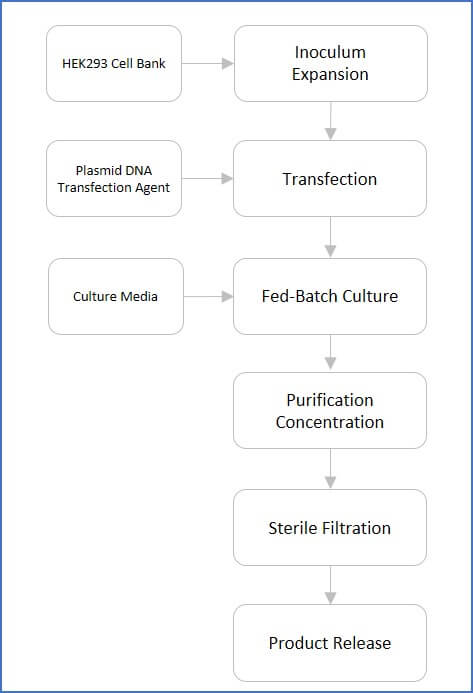
An Illustrated Flowchart for AAV