Transfer of cells into patients for medical purposes has been established as a viable treatment strategy for multiple disease areas including cancers. OranssiBio offers a wide range of technical development and manufacturing expertise across a variety types of cells including chimeric antigen receptor T cells (CAR-T), natural killer cells (NKs), and iPSCs.
At OranssiBio, our cell platform provides following features:
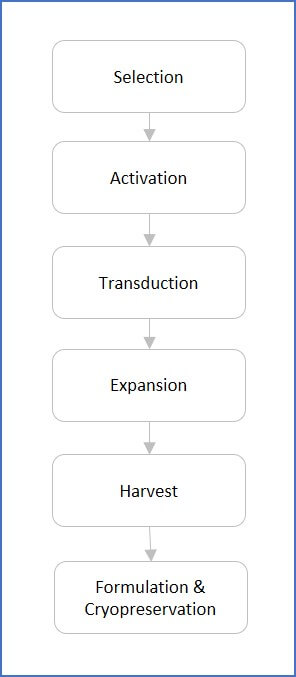
An Illustrated Flowchart for Cell