Lenti Virus (LV) is a powerful tool used in the field of gene therapy and gene editing. It can deliver large therapeutic payloads (up to 10kb) into target cells, either dividing or non-dividing ones, making it particularly useful for delivering genetic material into a wide range of cell types, including difficult-to-transfect or non-dividing cells.
At OranssiBio, we provide LV with the following features:
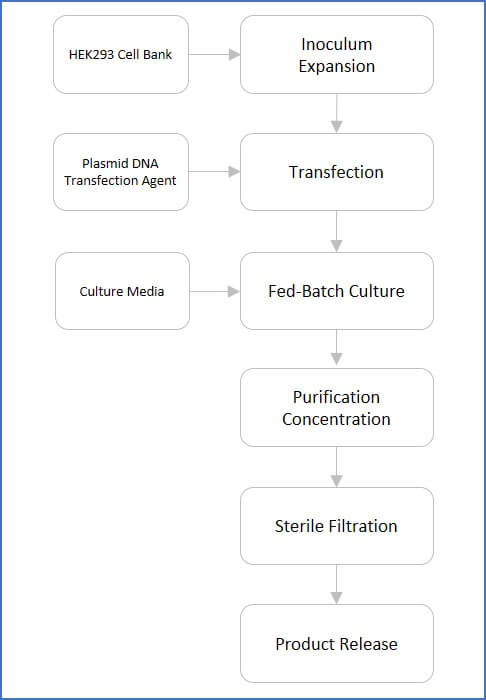
An Illustrated Flowchart for LV